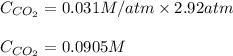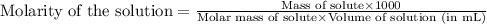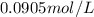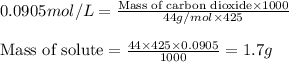Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which type of reaction always has an element and a compound as reactants
Answers: 1

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1
You know the right answer?
At the Henry's Law constant for carbon dioxide gas in water is . Calculate the mass in grams of gas...
Questions




Mathematics, 08.04.2020 20:16


Mathematics, 08.04.2020 20:16

History, 08.04.2020 20:16

Physics, 08.04.2020 20:16








Mathematics, 08.04.2020 20:16


Mathematics, 08.04.2020 20:16

Physics, 08.04.2020 20:16

Mathematics, 08.04.2020 20:16

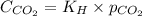
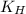 = Henry's constant =
= Henry's constant = 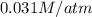
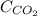 = molar solubility of carbon dioxide gas
= molar solubility of carbon dioxide gas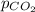 = partial pressure of carbon dioxide gas = 2.92 atm
= partial pressure of carbon dioxide gas = 2.92 atm