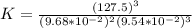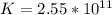For this heterogeneous system 2 A ( aq ) + 3 B ( g ) + C ( l ) − ⇀ ↽ − 2 D ( s ) + 3 E ( g ) the concentrations and pressures at equilibrium are [ A ] = 9.68 × 10 − 2 M , P B = 9.54 × 10 3 Pa , [ C ] = 14.64 M , [ D ] = 10.11 M , and P E = 9.56 × 10 4 torr . Calculate the thermodynamic equilibrium constant, K.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:40
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1

Chemistry, 22.06.2019 09:00
Which process does not require the presence of a physical substance in order to transfer heat? air in the atmosphere is heated by the ground. this warm air then rises, and cooler air falls. this is an example of what type of process? how is conduction different from radiation?
Answers: 1

Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

You know the right answer?
For this heterogeneous system 2 A ( aq ) + 3 B ( g ) + C ( l ) − ⇀ ↽ − 2 D ( s ) + 3 E ( g ) the con...
Questions




Health, 28.01.2020 14:49



Mathematics, 28.01.2020 14:49


Mathematics, 28.01.2020 14:49

Mathematics, 28.01.2020 14:49


Mathematics, 28.01.2020 14:49

Health, 28.01.2020 14:49


English, 28.01.2020 14:49

Chemistry, 28.01.2020 14:49

Mathematics, 28.01.2020 14:49

Mathematics, 28.01.2020 14:49



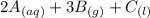 ⇄
⇄ 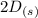

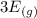
![[A] = 9.68*10^{-2}M](/tpl/images/0532/6642/3a7b2.png)
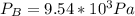
![[C]=14.64M](/tpl/images/0532/6642/fed12.png)
![[D]=10.11M](/tpl/images/0532/6642/c5c49.png)
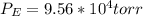
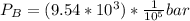
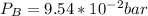
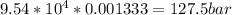
![K=\frac{[P_E]^3}{[A]^2[P_B]^3}](/tpl/images/0532/6642/53c7c.png)