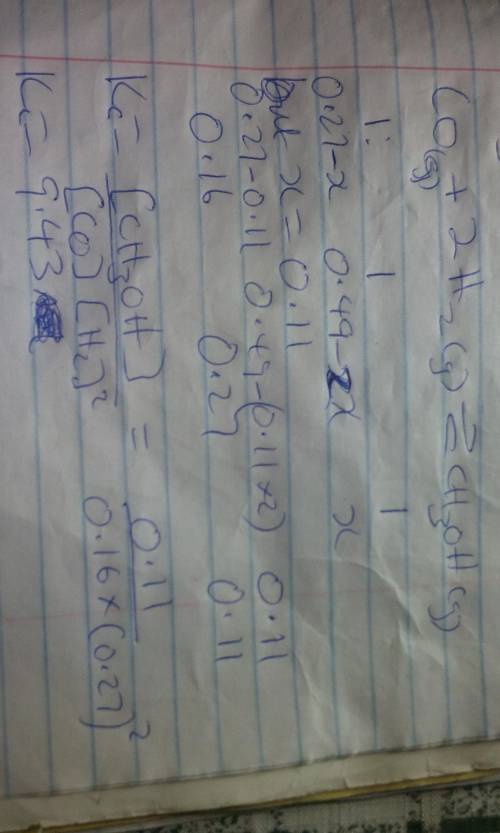Consider the following reaction:
CO(g)+2H2(g)⇌CH3OH(g)
This reaction is carr...

Consider the following reaction:
CO(g)+2H2(g)⇌CH3OH(g)
This reaction is carried out at a specific temperature with initial concentrations of [CO] = 0.27 M and [H2] = 0.49 M. At equilibrium, the concentration of CH3OH is 0.11 M. Find the equilibrium constant at this temperature.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Select the correct answer. given: 2libr + ba → babr2 + 2li in this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced? a. 1.18 mol b. 2.37 mol c. 4.73 mol d. 16.4 mol e. 32.9 mol
Answers: 2

Chemistry, 22.06.2019 07:40
The formation of a solid, also known as a is an indication of a chemical change. precipitate particulate particle powder
Answers: 3

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 22.06.2019 23:00
Condensation happens when water vapor cools. true or false? ?
Answers: 2
You know the right answer?
Questions

History, 25.11.2019 09:31


History, 25.11.2019 09:31

Chemistry, 25.11.2019 09:31

English, 25.11.2019 09:31

Mathematics, 25.11.2019 09:31


Mathematics, 25.11.2019 09:31

Chemistry, 25.11.2019 09:31

Chemistry, 25.11.2019 09:31


Mathematics, 25.11.2019 09:31

Mathematics, 25.11.2019 09:31

Mathematics, 25.11.2019 09:31

Mathematics, 25.11.2019 09:31



Business, 25.11.2019 09:31

Physics, 25.11.2019 09:31

Social Studies, 25.11.2019 09:31