
Chemistry, 04.03.2020 01:18 mdakane3772
The production capacity for acrylonitrile (C3H3N) in the United States exceeds 2 million pounds per year. Acrylonitrile, the building block for polyacrylonitrile fibers and a variety of plastics, is produced from gaseous propylene, ammonia, and oxygen.
2 C3H6(g) + 2 NH3(g) + 3 O2(g) → 2 C3H3N(g) + 6 H2O(g)
(a) What mass of acrylonitrile can be produced from a mixture of 1.20 kg of propylene (C3H6), 1.60 kg of ammonia, and 1.79 kg of oxygen, assuming 100% yield?
g
(b) What mass of water is produced?
g
Which starting materials are left in excess? (Select all that apply.)
propyleneammoniaoxygen
What mass of propylene is left in excess?
g
What mass of ammonia is left in excess?
g
What mass of oxygen is left in excess?
g

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:00
How is the atomic number of a nucleus changed by alpha decay
Answers: 2


Chemistry, 23.06.2019 03:30
In general metals get as you move from left to right across the periodic table.
Answers: 1

You know the right answer?
The production capacity for acrylonitrile (C3H3N) in the United States exceeds 2 million pounds per...
Questions




Mathematics, 13.02.2020 05:54

Mathematics, 13.02.2020 05:54






Chemistry, 13.02.2020 05:54


Mathematics, 13.02.2020 05:54


English, 13.02.2020 05:54

Mathematics, 13.02.2020 05:54


Biology, 13.02.2020 05:54


History, 13.02.2020 05:54

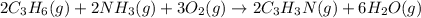
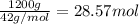
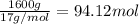
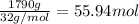
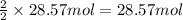 of ammonia
of ammonia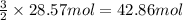 of oxygen gas
of oxygen gas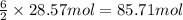 of water
of water

