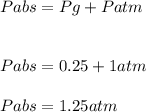Chemistry, 04.03.2020 03:27 akimadixon13
A ball has a volume of 5.27 liters and is at a temperature of 27.0°C. A pressure gauge attached to the ball reads 0.25 atmosphere. The atmospheric pressure is 1.00 atmosphere.
Calculate the absolute pressure inside the ball and the amount of air it contains.
The absolute pressure inside the ball is ??? atmospheres
The ball contains ???mole of air

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Turbo the snail moves across the ground at a pace of 12 feet per day. if the garden is 48 feet away, how many days will it take for the snail to get there?
Answers: 2

Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 11:00
Surface currents are caused by blank space . question 14 options: surface currents are caused by? differences in water temperature high salinity differences in density wind forces
Answers: 1
You know the right answer?
A ball has a volume of 5.27 liters and is at a temperature of 27.0°C. A pressure gauge attached to t...
Questions

Chemistry, 04.10.2020 05:01

Health, 04.10.2020 05:01


Mathematics, 04.10.2020 05:01

English, 04.10.2020 05:01



Computers and Technology, 04.10.2020 05:01

Mathematics, 04.10.2020 05:01



English, 04.10.2020 05:01

Mathematics, 04.10.2020 05:01

Biology, 04.10.2020 05:01

Social Studies, 04.10.2020 05:01

English, 04.10.2020 05:01



Mathematics, 04.10.2020 05:01