
Chemistry, 04.03.2020 22:12 doggylover8655
You want to determine the protein content in milk with the Kjeldahl method. You take 100 g whole milk and use 100 mL of 0.5 M hydrochloric acid to collect ammonia. You needed 34.50 mL of 0.3512 M NaOH for the back-titration. Calculate the percentage of protein in the sample?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
This large tectonic plate is bounded on three sides by whats know as the ring of fire. what is the name of this tectonic plate? a) pacific plate b) eurasian plate c) north american plate d) indo- australian plate plz it's science but there's no option for science so i picked chemistry
Answers: 2


Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2
You know the right answer?
You want to determine the protein content in milk with the Kjeldahl method. You take 100 g whole mil...
Questions




Mathematics, 21.06.2020 03:57



Mathematics, 21.06.2020 03:57







Mathematics, 21.06.2020 03:57






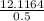 = 24.2328 mL
= 24.2328 mL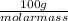
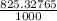 × 100
× 100 

