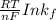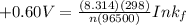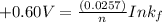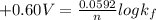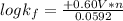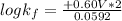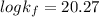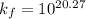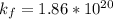Chemistry, 04.03.2020 22:59 emilyanneK236
Be sure to answer all parts. Zinc is an amphoteric metal, meaning it reacts with both acids and bases. The standard reduction potential is −1.36 V for the following reaction: (1)Zn(OH)42−(aq) + 2e− → Zn(s) + 4OH−(aq) Calculate the formation constant Kf for the reaction: (2)Zn2+(aq) + 4OH−(aq) ⇌ Zn(OH)42−(aq)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Select the correct answer. which statement is true about a polarized object? o a. it gains electrons and becomes negatively charged. ob. it gains protons and becomes positively charged. oc. the number of positive and negative charges can be the same. od. it has to be a metal. o e. there is no change in the distribution of the charge in the object. reset next what
Answers: 3

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1
You know the right answer?
Be sure to answer all parts. Zinc is an amphoteric metal, meaning it reacts with both acids and base...
Questions



Computers and Technology, 14.01.2021 23:00

Mathematics, 14.01.2021 23:00

Mathematics, 14.01.2021 23:00


Mathematics, 14.01.2021 23:00

Mathematics, 14.01.2021 23:00



English, 14.01.2021 23:00

Advanced Placement (AP), 14.01.2021 23:10


Mathematics, 14.01.2021 23:10



Mathematics, 14.01.2021 23:10

Mathematics, 14.01.2021 23:10

Mathematics, 14.01.2021 23:10


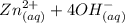 ⇄
⇄ 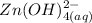

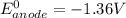

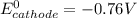
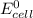 is given as:
is given as: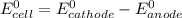
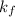 of the
of the