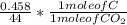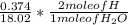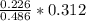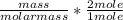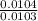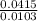Chemistry, 05.03.2020 00:53 hbhdnjdndj1867
Dimethylhydrazine is a carbon-hydrogen-nitrogen compound used in rocket fuels. When burned in an excess of oxygen, a 0.312 gg sample yields 0.458 gg CO2CO2 and 0.374 gg H2OH2O. The nitrogen content of a 0.486 gg sample is converted to 0.226 gg N2N2.What is the empirical formula of dimethylhydrazine?Express your answer as a chemical formula.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:40
Base your answer on the information below and on your knowledge of chemistry. nitrogen dioxide, no2, is a dark brown gas that is used to make nitric acid and to bleach flour. nitrogen dioxide has a boiling point of 294 k at 101.3 kpa. in a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, n2o4. this equilibrium is represented by the equation below. 2no2(g) n2o4(g) + 58kj at standard pressure, compare the strength of intermolecular forces in no2(g) to the strength of intermolecular forces in n2(g).
Answers: 2

Chemistry, 22.06.2019 05:40
Salicylic acid is a very important acid. it is used to synthesize the aspirin by treating with acetic anhydride. a 0.2015-g sample of salicylic acid was dissolved in a 100.00-ml volumetric flask, and the solution was diluted to the mark. a 10-ml aliquot of this solution was titrated with standard naoh (0.01130 + 0.2% n) to a phenolphthalein faint pink color end point at 19.81 ml. (a) (calculate the normality of the salicylic acid solution used in the titration. (b) assuming the salicylic acid is pure, what is the equivalent weight of the salicylic acid? practice problems for the final exam (continued) (c) (calculate the inherent error in the determination of the equivalent weight you calculated in part (b). use the following absolute errors in the equipment /glassware when calculating the inherent error. 5.00-ml pipet: + 0.02 ml 100-ml volumetric flask: + 0.08 ml analytical balance: + 0.2 mg 25-ml buret: + 0.03 ml
Answers: 2

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1
You know the right answer?
Dimethylhydrazine is a carbon-hydrogen-nitrogen compound used in rocket fuels. When burned in an exc...
Questions


Computers and Technology, 02.06.2021 22:50

Mathematics, 02.06.2021 22:50

Mathematics, 02.06.2021 22:50




Mathematics, 02.06.2021 22:50

Social Studies, 02.06.2021 22:50

Mathematics, 02.06.2021 22:50

History, 02.06.2021 22:50

Chemistry, 02.06.2021 22:50

Computers and Technology, 02.06.2021 22:50

Spanish, 02.06.2021 22:50

Mathematics, 02.06.2021 22:50

Mathematics, 02.06.2021 22:50

Mathematics, 02.06.2021 22:50



History, 02.06.2021 22:50