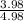Chemistry, 04.03.2020 23:17 jayleneeurich
A 0.10 M imidazole solution has a pH of 6.6. To the nearest hundredth of a unit, what fraction of the molecules are in the neutral (imidazole) form? (The pKa of the imidazolium ion is 6.0.)

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:40
What are the resulting coefficients when you balance the chemical equation for the combustion of ethane, c2h6? in this reaction, ethane is burned in the presence of oxygen (o2) to form carbon dioxide (co2) and water (h2o). (g)+(g)→(g)+(g)
Answers: 1

Chemistry, 21.06.2019 23:10
Which statement describes both homogeneous mixtures and heterogeneous mixtures?
Answers: 1

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2

Chemistry, 22.06.2019 19:40
Scientists have developed an explanation of a phenomenon from several verified hypotheses. the explanation has been confirmed through numerous experimental tests.which option best describes this explanation? a. scientific lawb. research questionc. hypothesisd. scientific theory
Answers: 3
You know the right answer?
A 0.10 M imidazole solution has a pH of 6.6. To the nearest hundredth of a unit, what fraction of th...
Questions

Social Studies, 29.08.2019 21:30

Mathematics, 29.08.2019 21:30




Mathematics, 29.08.2019 21:30


Mathematics, 29.08.2019 21:30



Social Studies, 29.08.2019 21:30

Mathematics, 29.08.2019 21:30



Biology, 29.08.2019 21:30


English, 29.08.2019 21:30

Biology, 29.08.2019 21:30


Mathematics, 29.08.2019 21:30

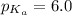
![pH=pK_a+\log \frac{[Salt]}{[Acid]}](/tpl/images/0533/8426/e961a.png)
![pH=pK_a+\log \frac{[\text{Imidazole}]}{[\text{Imidazolium ion}]}](/tpl/images/0533/8426/c2e7e.png)
![6.6=6.0+\log \frac{[\text{Imidazole}]}{[\text{Imidazolium ion}]}](/tpl/images/0533/8426/895b0.png)
![\frac{[\text{Imidazole}]}{[\text{Imidazolium ion}]}=10^{6.6-6.0}](/tpl/images/0533/8426/0952e.png)
![\frac{[\text{Imidazole}]}{[\text{Imidazolium ion}]}=10^{0.6}](/tpl/images/0533/8426/2864b.png)
![\frac{[\text{Imidazole}]}{[\text{Imidazolium ion}]}=3.98](/tpl/images/0533/8426/0af59.png)
![[\text{Imidazole}]=3.98[\text{Imidazolium ion}]](/tpl/images/0533/8426/f647e.png) ...........(1)
...........(1)![\frac{[\text{Imidazole}]}{[\text{Imidazole}]+[\text{Imidazolium ion}]}](/tpl/images/0533/8426/affc2.png)
![\frac{3.98[\text{Imidazolium ion}]}{3.98[\text{Imidazolium ion}]+[\text{Imidazolium ion}]}](/tpl/images/0533/8426/250f9.png)
![\frac{3.98[\text{Imidazolium ion}]}{4.98[\text{Imidazolium ion}]}](/tpl/images/0533/8426/6749d.png)