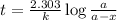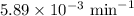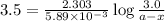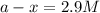Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 13:30
What phase of matter has particles that are held together but can flow past each other and takes the shape of a container, filling it from the bottom up?
Answers: 1

Chemistry, 21.06.2019 15:30
If 200.0g of copper(ll) sulfate react with an excess of zinc metal, what is the theoretical yield of copper
Answers: 1

Chemistry, 22.06.2019 07:00
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2

Chemistry, 22.06.2019 13:30
Astudent is trying to create a table that compares hypotheses, theories, and laws. hypothesis theory law do scientific researchers formulate it? yes yes yes does it explain why things happen? yes yes no yes yes yes is it used to make predictions? no yes yes which of the following questions would most likely fill the blank in the table? is it an intelligent guess? is it newly formulated? is it based on observations? has it been proved?
Answers: 1
You know the right answer?
N2O5 decomposes to form NO2 and O2 with first-order kinetics. The initial concentration of N2O5 is 3...
Questions




Chemistry, 19.06.2021 21:30

Mathematics, 19.06.2021 21:30

English, 19.06.2021 21:30


Mathematics, 19.06.2021 21:30

Mathematics, 19.06.2021 21:30

Mathematics, 19.06.2021 21:30

Mathematics, 19.06.2021 21:30

Mathematics, 19.06.2021 21:30

Mathematics, 19.06.2021 21:30


English, 19.06.2021 21:30


Mathematics, 19.06.2021 21:30

English, 19.06.2021 21:40


History, 19.06.2021 21:40

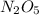 is, 2.9 M
is, 2.9 M