
Chemistry, 04.03.2020 23:26 TheOneandOnly003
A compound is 92.2% Carbon and 7.76% Hydrogen. The formula mass of the compound is 78.1 g. Calculate the empirical formula and molecular formula of the compound.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 16:30
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1

Chemistry, 22.06.2019 17:30
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2

You know the right answer?
A compound is 92.2% Carbon and 7.76% Hydrogen. The formula mass of the compound is 78.1 g. Calculate...
Questions

Biology, 16.09.2021 05:50


Social Studies, 16.09.2021 05:50


Mathematics, 16.09.2021 05:50

Mathematics, 16.09.2021 05:50




Mathematics, 16.09.2021 05:50


English, 16.09.2021 05:50

Mathematics, 16.09.2021 05:50

Mathematics, 16.09.2021 05:50



Physics, 16.09.2021 06:00

Mathematics, 16.09.2021 06:00


Social Studies, 16.09.2021 06:00

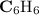
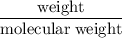
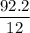

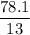
 .
.

