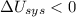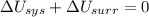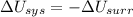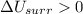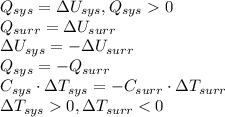Chemistry, 04.03.2020 23:29 isaiahcannon5709
Determine if each of the statements is True or False regarding the First Law of Thermodynamics.
1. If the system loses energy to the surroundings, the surroundings could also lose energy to the system.
2. If the system gains thermal energy from the surroundings, the temperature of the surroundings decreases.
3 If the system gains 25 kJ of energy from the surroundings without doing any work on the surroundings, the surroundings could lose 20 kJ of energy.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 10:40
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1
You know the right answer?
Determine if each of the statements is True or False regarding the First Law of Thermodynamics.
Questions

Mathematics, 05.03.2021 01:00


Physics, 05.03.2021 01:00

History, 05.03.2021 01:00

Biology, 05.03.2021 01:00



Mathematics, 05.03.2021 01:00


Mathematics, 05.03.2021 01:00


Mathematics, 05.03.2021 01:00



Biology, 05.03.2021 01:00

Geography, 05.03.2021 01:00


Mathematics, 05.03.2021 01:00


Mathematics, 05.03.2021 01:00