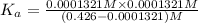Chemistry, 05.03.2020 03:06 jacqueline398
In the laboratory, a general chemistry student measured the pH of a 0.426 M aqueous solution of hypochlorous acid to be 3.897. Use the information she obtained to determine the Ka for this acid. Ka(experiment)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:30
If the root word engage means “to connect with something,” what does the word disengage mean in the following sentence? he disengaged the gears by stepping on the clutch pedal.a.added more engine powerb.activated a connection to the pedalc.stalled the engined.released a connection to the pedal
Answers: 1

Chemistry, 22.06.2019 02:30
When svante arrhenius first proposed his acid-base theory, he was a doctoral candidate. his professors thought his ideas were unfounded. within a decade, the arrhenius theory of acid-base was widely accepted and praised within the scientific world. arrhenius defined acids as compounds having ionizable hydrogen and bases as compounds with ionizable a) barium. b) hydronium. c) hydroxide. d) oxygen.
Answers: 3

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 07:30
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3
You know the right answer?
In the laboratory, a general chemistry student measured the pH of a 0.426 M aqueous solution of hypo...
Questions








History, 31.05.2020 00:00

Mathematics, 31.05.2020 00:00

History, 31.05.2020 00:00


Mathematics, 31.05.2020 00:00

Physics, 31.05.2020 00:00






Mathematics, 31.05.2020 00:01

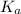 of the acid is :
of the acid is :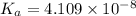
![[H^+]](/tpl/images/0534/0971/07acb.png)
![pH=-\log[H^+]](/tpl/images/0534/0971/cf945.png)
![3.879=-\log[H^+]](/tpl/images/0534/0971/71ded.png)
![[H^+]=10^{-3.876}=0.0001321 M](/tpl/images/0534/0971/1f1ff.png)
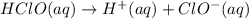
![K_a=\frac{[H^+][ClO^-]}{[HClO]}](/tpl/images/0534/0971/63c9f.png)