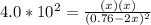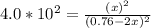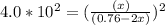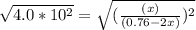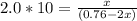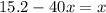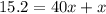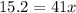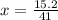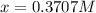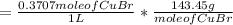Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 08:00
Match the mixture with the substance// i really need on this guys (it’s a pic btw)
Answers: 1

Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2
You know the right answer?
Copper(I) ions in aqueous solution react with NH 3 ( aq ) according to Cu + ( aq ) + 2 NH 3 ( aq ) ⟶...
Questions

Social Studies, 11.10.2021 22:00


Biology, 11.10.2021 22:00


History, 11.10.2021 22:00

Mathematics, 11.10.2021 22:00

Mathematics, 11.10.2021 22:00

Biology, 11.10.2021 22:00

Mathematics, 11.10.2021 22:00

Mathematics, 11.10.2021 22:00


Mathematics, 11.10.2021 22:00


Chemistry, 11.10.2021 22:00


English, 11.10.2021 22:00

Mathematics, 11.10.2021 22:00

Mathematics, 11.10.2021 22:00


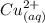

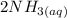

![[Cu(NH_3)_2]^+_{(aq)}](/tpl/images/0534/1149/6f7e0.png) ------equation (1)
------equation (1)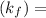
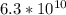
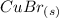 yields:
yields: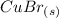 ⇄
⇄ 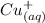
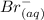 -------------- equation (2)
-------------- equation (2)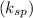

![\frac{[[Cu(NH_3)_2]^+]}{[Cu^{2+}][NH_3]^{2}}](/tpl/images/0534/1149/99ab3.png) --------- equation (3)
--------- equation (3)![= [Cu^+][Br^-]](/tpl/images/0534/1149/1d1b7.png) --------- equation (4)
--------- equation (4)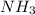 , the net reaction for
, the net reaction for 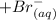

![\frac{[[Cu(NH_3)_2]^+][Br^-]}{[NH_3]^2}](/tpl/images/0534/1149/25fcf.png)
![[Cu^+]](/tpl/images/0534/1149/41578.png) ; we have:
; we have:![\frac{[[Cu(NH_3)_2]^+][Br^-]}{[NH_3]^2}*\frac{[Cu^+]}{[Cu^+]}](/tpl/images/0534/1149/7861d.png)
![\frac{[[Cu(NH_3)_2]^+}{[NH_3]^2[Cu^+]}*{[Cu^+][Br^-]}](/tpl/images/0534/1149/49395.png)
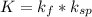
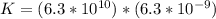
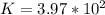
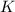 ≅
≅