
Chemistry, 05.03.2020 03:40 joThompson
When this system is at equilibrium at a certain temperature PCl5(g) ⇋ PCl3(g) + Cl2(g), the concentrations are found to be [PCl5] = 0.40 M, [PCl3] = [Cl2] = 0.20. If the volume of the container is suddenly halved at the same temperature, what will be the new equilibrium concentration of PCl5?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:30
Over the last 90 years, scientists have added to the body of evidence supporting the big bang theory. what is the latest piece of evidence discovered in 2014?
Answers: 1

Chemistry, 22.06.2019 10:20
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3

Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1

Chemistry, 22.06.2019 19:30
What is the area in square meters of 448 g ai foil that has a thickness of 23921 nm? the density is 2.70 g/cm
Answers: 3
You know the right answer?
When this system is at equilibrium at a certain temperature PCl5(g) ⇋ PCl3(g) + Cl2(g), the concentr...
Questions

Mathematics, 24.05.2020 00:06


Mathematics, 24.05.2020 00:06


Social Studies, 24.05.2020 00:06

Mathematics, 24.05.2020 00:06

Mathematics, 24.05.2020 00:06



History, 24.05.2020 00:06

Mathematics, 24.05.2020 00:06

History, 24.05.2020 00:06


Mathematics, 24.05.2020 00:06




Mathematics, 24.05.2020 00:06

Mathematics, 24.05.2020 00:06

Mathematics, 24.05.2020 00:06

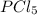 will be 0.9 M.
will be 0.9 M.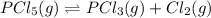
![[PCl_5]=0.40 M](/tpl/images/0534/1154/cb9c6.png)
![[PCl_3]=[Cl_2]=0.20 M](/tpl/images/0534/1154/9bc6a.png)
![K_c=\frac{[PCl_3][Cl_2]}{[PCl_5]}](/tpl/images/0534/1154/73fe0.png)
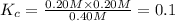
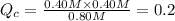
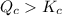
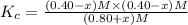
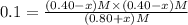
![[PCl_5]=(0.80+x) M=(0.80+0.1) M = 0.90](/tpl/images/0534/1154/dbad2.png)


