
Chemistry, 05.03.2020 05:16 calmicaela12s
Consider the unbalanced equation for the combustion of hexane: C6H14 (g) O2 (g) --> CO2 (g) H2O (g) Balance the equation and determine how many moles of O2 are required to react completely with 7.2 moles of C6H14.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:00
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2


Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1

You know the right answer?
Consider the unbalanced equation for the combustion of hexane: C6H14 (g) O2 (g) --> CO2 (g) H2O (...
Questions


History, 19.09.2019 09:30

History, 19.09.2019 09:30


Social Studies, 19.09.2019 09:30

Mathematics, 19.09.2019 09:30

English, 19.09.2019 09:30


Social Studies, 19.09.2019 09:30


Biology, 19.09.2019 09:30






History, 19.09.2019 09:30



English, 19.09.2019 09:30

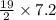 .
.

