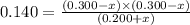Consider mixture C, which will cause the net reaction to proceed in reverse. Concentration (M)initial:change:equilibrium:[XY]0 .200+x0.200+x←net⇌[X]0.300−x0.300−x +[Y]0.300−x0.300−x The change in concentration, x, is positive for the reactants because they are produced and negative for the products because they are consumed.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Balance this equation: n2 + h2 > nh3, write the following molar ratios: n2 / n2 / nh3 h2 /
Answers: 1

Chemistry, 22.06.2019 12:00
the mississippians were considered to be horticulturalists, which means they were
Answers: 1

Chemistry, 23.06.2019 07:00
0.88 moles of n2o5 (g) was placed in a sealed 1.00 l vessel. calculate the equilibrium concentration of n2o5. no2, and o2 and the equilibrium constant after equilibrium has been reached by 65.0% of the n2o5 decomposing.
Answers: 1

Chemistry, 23.06.2019 08:00
What is the temperature in kelvin of a gas if it is allowed to expand from 1.50 l to 4.50 l? the initial temperature is 10.0°c and pressure is constant throughout the change. which equation should you use? t2= v2/v1 t1 what is the final temperature? ⇒ 849 k these are the answers.
Answers: 1
You know the right answer?
Consider mixture C, which will cause the net reaction to proceed in reverse. Concentration (M)initia...
Questions


Mathematics, 28.02.2021 22:40


Mathematics, 28.02.2021 22:40


Mathematics, 28.02.2021 22:40

Computers and Technology, 28.02.2021 22:40

History, 28.02.2021 22:40

History, 28.02.2021 22:40



Mathematics, 28.02.2021 22:40





Mathematics, 28.02.2021 22:40

Mathematics, 28.02.2021 22:40


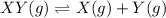
![K_c=\farc{[X][Y]}{[XY]}](/tpl/images/0534/4698/91cd9.png)