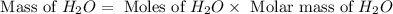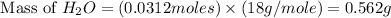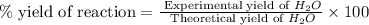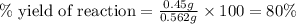Chemistry, 05.03.2020 13:09 vanessacasillas452
The combustion of glucose is represented by the following balanced equation: C6H12O6+6 O2→6 H2O+6 CO2. The reaction uses 1 gram of both C6H12O6 and O2. What is the percent yield if 0.45 g of H2O is produced? a 0.558% b 100% c 0.31% d 80%

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2

Chemistry, 22.06.2019 19:30
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1

Chemistry, 22.06.2019 21:30
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1
You know the right answer?
The combustion of glucose is represented by the following balanced equation: C6H12O6+6 O2→6 H2O+6 CO...
Questions

Business, 15.02.2022 14:00


Mathematics, 15.02.2022 14:00


History, 15.02.2022 14:00

Chemistry, 15.02.2022 14:00





Mathematics, 15.02.2022 14:00

Physics, 15.02.2022 14:00

Mathematics, 15.02.2022 14:00






Geography, 15.02.2022 14:00

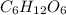 = 1 g
= 1 g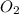 = 1 g
= 1 g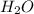 = 18 g/mole
= 18 g/mole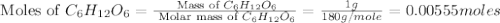
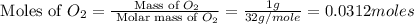
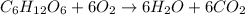
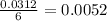 moles of
moles of