Answers: 1
Another question on Chemistry



Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3

Chemistry, 23.06.2019 00:30
Maya wrote if you step to describe how carbon circulates between the atmosphere and living organisms
Answers: 1
You know the right answer?
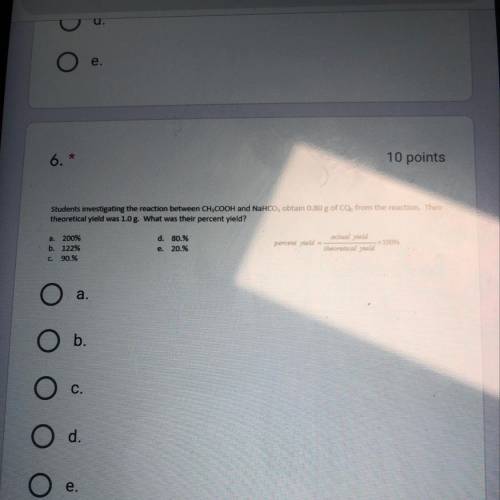
Students investigating the reaction between CH3COOH and NaHCO3 obtain .80 g of CO2 from the reaction...
Questions


Mathematics, 28.12.2020 05:40


Social Studies, 28.12.2020 05:40

English, 28.12.2020 05:40

Mathematics, 28.12.2020 05:40




Mathematics, 28.12.2020 05:40

Physics, 28.12.2020 05:40


Mathematics, 28.12.2020 05:40

Physics, 28.12.2020 05:40

English, 28.12.2020 05:40


History, 28.12.2020 05:40

Biology, 28.12.2020 05:40

English, 28.12.2020 05:50

Social Studies, 28.12.2020 05:50