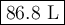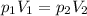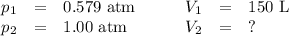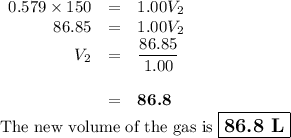Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1


Chemistry, 23.06.2019 01:30
Some molecular compounds, such as hcl, ionize completely in solution. for molecular compounds such as h2co3, most molecules do not ionize in solution. which describes the properties of these two solutes? a. hcl and h2co3 have the same effect on the properties of the solution. b. hcl raises the freezing point of water more than h2co3 does. c. hcl raises the boiling point of water more than h2co3 does.
Answers: 2

Chemistry, 23.06.2019 03:50
How many liters of oxygen gas, at standardtemperature and pressure, will react with 35.8 grams ofiron metal? 4 fe (s) + 3 o2 (g) → 2 fe2o3 (s)
Answers: 3
You know the right answer?
A pump contains 1.5 L of air at 175 kPa. You draw back on the piston of the pump, expanding the volu...
Questions

Mathematics, 21.11.2020 21:50

Mathematics, 21.11.2020 21:50


Mathematics, 21.11.2020 21:50


Mathematics, 21.11.2020 21:50


Mathematics, 21.11.2020 21:50


Mathematics, 21.11.2020 21:50


Mathematics, 21.11.2020 22:00




Mathematics, 21.11.2020 22:00

English, 21.11.2020 22:00


Engineering, 21.11.2020 22:00