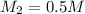Chemistry, 06.03.2020 01:58 airsealands
A 10.0 mL 10.0 mL aliquot is removed from the described stock solution and diluted to a total volume of 100.0 mL. 100.0 mL. Calculate the molarity of the dilute solution.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:30
What is the value of keq for the reaction expressed in scientific notation
Answers: 1

Chemistry, 22.06.2019 02:30
Select each correct answer. more than one answer may be correct. which of the following is a characteristic of unicellular organisms? they can possess tissues and organs. all of their functions are performed by a single cell. they are usually microscopic. each of their cells is specialized to perform a specific function.
Answers: 1

Chemistry, 22.06.2019 04:00
What layer of the atmosphere is directly above the troposphere?
Answers: 1

Chemistry, 22.06.2019 06:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1
You know the right answer?
A 10.0 mL 10.0 mL aliquot is removed from the described stock solution and diluted to a total volume...
Questions

Mathematics, 01.06.2021 16:40



Geography, 01.06.2021 16:40


Mathematics, 01.06.2021 16:40


History, 01.06.2021 16:40

Mathematics, 01.06.2021 16:40


Mathematics, 01.06.2021 16:40


Chemistry, 01.06.2021 16:40

Mathematics, 01.06.2021 16:40


Chemistry, 01.06.2021 16:40

English, 01.06.2021 16:40



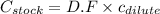
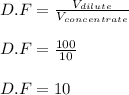
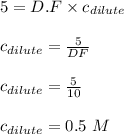
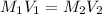
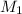 = molarity of stock solution = 5 M
= molarity of stock solution = 5 M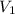 = volume of stock solution = 10.0 ml ml
= volume of stock solution = 10.0 ml ml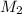 = molarity of dilute solution = ?
= molarity of dilute solution = ?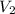 = volume of dilute solution = 100.0 ml
= volume of dilute solution = 100.0 ml