Chemistry, 06.03.2020 01:48 GhostElite6383
Ammonia will decompose into nitrogen and hydrogen at high temperature. An industrial chemist studying this reaction fills a 7.50 l tank with 29.0 mol of ammonia gas at 35.0 °C. She then raises the temperature, and when the mixture has come to equilibrium measures the amount of nitrogen gas to be 13.0 mol.
Calculate the concentration equilibrium constant for the decomposition of ammonia at the final temperature of the mixture. Round your answer to significant digits.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 15:30
Which mathematical relationship should you us to convert moles of a substance into grams
Answers: 1

Chemistry, 22.06.2019 05:30
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease.correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1

Chemistry, 22.06.2019 14:30
Ahypothesis must be testable and falsifiable to be considered scientific a. trueb. false
Answers: 1

Chemistry, 22.06.2019 21:00
Acandle’s wick is the fabric string that holds the flame, and it burns down at a constant slow pace when the candle is lit. the wick is usually surrounded by wax. which is the most important property of covalent compounds that makes them useful for making candle wax? a low boiling point a low melting point a high boiling point a high melting point
Answers: 1
You know the right answer?
Ammonia will decompose into nitrogen and hydrogen at high temperature. An industrial chemist studyin...
Questions

English, 06.04.2021 02:50


Mathematics, 06.04.2021 03:00



Arts, 06.04.2021 03:00




Biology, 06.04.2021 03:00

Mathematics, 06.04.2021 03:00

Mathematics, 06.04.2021 03:00

Mathematics, 06.04.2021 03:00

Physics, 06.04.2021 03:00

Mathematics, 06.04.2021 03:00


Physics, 06.04.2021 03:00



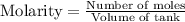
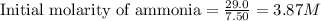
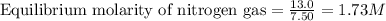
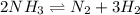
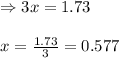
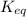 for above equation follows:
for above equation follows:![K_{eq}=\frac{[N_2][H_2]^3}{[NH_3]^2}](/tpl/images/0535/0025/804f3.png)