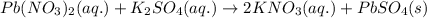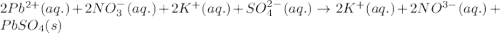Chemistry, 06.03.2020 06:22 derekcalhoun4943
Enter the complete ionic equation to show the reaction of aqueous lead(II) nitrate with aqueous potassium sulfate to form solid lead(II) sulfate and aqueous potassium nitrate. Express your answer as a chemical equation. Identify all of the phases in your answer. nothing

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2

Chemistry, 22.06.2019 15:30
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks.energy was destroyed inside the blocks.energy was absorbed into the blocks from outside the system.energy was transferred from the warmer block to the cooler block.
Answers: 2

Chemistry, 22.06.2019 20:20
Which symbol can be used to indicate the pressure at which a chemical reaction is carried out? 25°c 2 atm pa
Answers: 2

Chemistry, 23.06.2019 01:00
You wish to prepare a buffer consisting of acetic acid and sodium acetate with a total acetic acetate plus acetate concentration of 250 mm and a ph of 5. what concentrations of acetic acid and sodium acetate should you use
Answers: 1
You know the right answer?
Enter the complete ionic equation to show the reaction of aqueous lead(II) nitrate with aqueous pota...
Questions

Chemistry, 30.05.2021 07:40

History, 30.05.2021 07:40

Mathematics, 30.05.2021 07:40

History, 30.05.2021 07:40

Mathematics, 30.05.2021 07:40


Mathematics, 30.05.2021 07:40

Mathematics, 30.05.2021 07:40

Health, 30.05.2021 07:40

Mathematics, 30.05.2021 07:40

Social Studies, 30.05.2021 07:40

Social Studies, 30.05.2021 07:40

Mathematics, 30.05.2021 07:40

Biology, 30.05.2021 07:40


Mathematics, 30.05.2021 07:40


Mathematics, 30.05.2021 07:40

Mathematics, 30.05.2021 07:40

Chemistry, 30.05.2021 07:40