
Chemistry, 06.03.2020 07:25 cjacobs77311
Sulfur and fluorine react in a combination reaction to produce sulfur hexafluoride: S (s) 3F 2(g) SF 6 (g) The maximum amount of SF 6 that can be produced from the reaction of 3.5 g of sulfur with of fluorine is g.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:30
What’s the scientific notation for the number 6,840,000,000
Answers: 1

Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3

Chemistry, 22.06.2019 20:20
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3

You know the right answer?
Sulfur and fluorine react in a combination reaction to produce sulfur hexafluoride: S (s) 3F 2(g) SF...
Questions

History, 09.10.2019 23:00

Mathematics, 09.10.2019 23:00


Physics, 09.10.2019 23:00



Mathematics, 09.10.2019 23:00

History, 09.10.2019 23:00

Social Studies, 09.10.2019 23:00

Mathematics, 09.10.2019 23:00




English, 09.10.2019 23:00


History, 09.10.2019 23:00

History, 09.10.2019 23:00


Mathematics, 09.10.2019 23:00

History, 09.10.2019 23:00

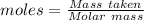
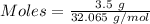
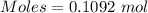
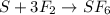
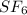 that can be produced from the reaction of 3.5 g of sulfur with of fluorine.
that can be produced from the reaction of 3.5 g of sulfur with of fluorine.

