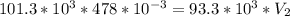Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3

Chemistry, 23.06.2019 03:00
The size (radius) of an oxygen molecule is about 2.0 ×10−10m. make a rough estimate of the pressure at which the finite volume of the molecules should cause noticeable deviations from ideal-gas behavior at ordinary temperatures (t= 300k ). assume that deviatons would be noticeable when volume of the gas per molecule equals the volume of the molecule itself.
Answers: 3
You know the right answer?
A sample of neon occupies a volume of 478 mL at STP. What will be the volume of the neon when the pr...
Questions

Mathematics, 21.01.2021 21:30



Mathematics, 21.01.2021 21:30

Computers and Technology, 21.01.2021 21:30

Mathematics, 21.01.2021 21:30

Mathematics, 21.01.2021 21:30

Mathematics, 21.01.2021 21:30

Mathematics, 21.01.2021 21:30

English, 21.01.2021 21:30

Biology, 21.01.2021 21:30






Mathematics, 21.01.2021 21:30

Mathematics, 21.01.2021 21:30


Biology, 21.01.2021 21:30

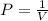
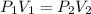
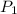 is 1 atm or 101.3 kPa and the initial volume is 478 mL. Similarly, the final pressure is 93.3 kPa and the final volume will be
is 1 atm or 101.3 kPa and the initial volume is 478 mL. Similarly, the final pressure is 93.3 kPa and the final volume will be