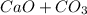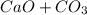Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 14:20
Calculate the enthalpy of the following reaction: 4 b (s) + 3 o2 (g) → 2 b2o3 (s) given the following pertinent information: (a) b2o3 (s) + 3 h2o (g) → 3 o2 (g) + b2h6 (g), δhoa = +2035 kj (b) 2 b (s) + 3 h2 (g) → b2h6 (g), δhob = +36 kj (c) h2 (g) + latex: \frac{1}{2} 1 2 o2 (g) → h2o (l), δhoc = −285 kj (d) h2o (l) → h2o (g), δhod = +44 kj
Answers: 3

Chemistry, 22.06.2019 06:20
If i can still dissolve more sugar into the solution at a certain temperature what would i call that solution
Answers: 3

Chemistry, 22.06.2019 16:00
As changes in energy levels of electrons increase, the frequencies of atomic line spectra they emit
Answers: 2

Chemistry, 22.06.2019 18:50
At stp, which substance is the best conductor of electricity? a. nitrogen b. neon c. sulfur d. silver
Answers: 1
You know the right answer?
Problem Page Question Solid calcium carbonate decomposes into solid calcium oxide and carbon dioxide...
Questions



Mathematics, 21.01.2021 18:40


Mathematics, 21.01.2021 18:40

Health, 21.01.2021 18:40

Mathematics, 21.01.2021 18:40

Chemistry, 21.01.2021 18:40

Spanish, 21.01.2021 18:40




Mathematics, 21.01.2021 18:40



Computers and Technology, 21.01.2021 18:40

Biology, 21.01.2021 18:40



Mathematics, 21.01.2021 18:40

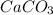 →
→