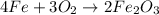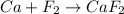Chemistry, 29.08.2019 04:30 thompsonhomes1
Classify the following reactions as either redox, acid-base, or precipitation. a) (cl2) + (2oh-) --> (cl-) + (clo-) + (h2o). b) already got. c) (nh3) + (h+) --> (nh4+). d) (4fe) + (3o2) --> (2fe2o3). e) (ca) + (f2) --> (caf2)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:20
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 22.06.2019 07:30
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 08:40
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1
You know the right answer?
Classify the following reactions as either redox, acid-base, or precipitation. a) (cl2) + (2oh-) --&...
Questions



Physics, 28.04.2021 03:10




Computers and Technology, 28.04.2021 03:10





Chemistry, 28.04.2021 03:10

Mathematics, 28.04.2021 03:10






Mathematics, 28.04.2021 03:10

Geography, 28.04.2021 03:10

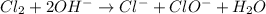
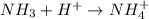
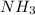 is a base and
is a base and 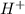 is an acid react to give
is an acid react to give 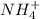 as a salt.
as a salt.