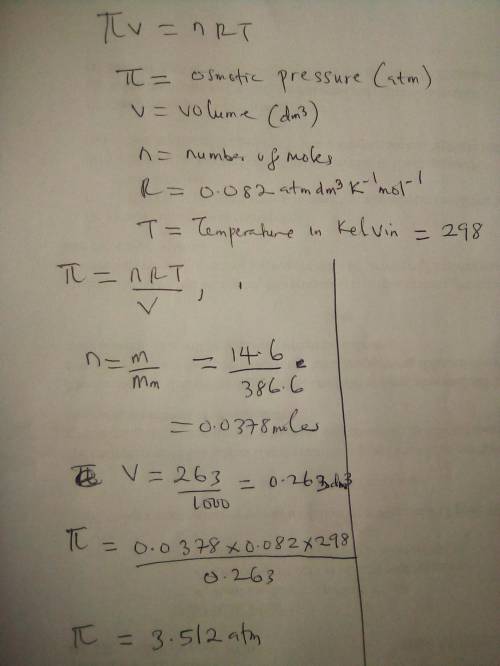Chemistry, 06.03.2020 19:06 teamzomaxx6584
The nonvolatile, nonelectrolyte cholesterol, C27H46O (386.60 g/mol), is soluble in benzene C6H6. Calculate the osmotic pressure generated when 14.6 grams of cholesterol are dissolved in 263 ml of a benzene solution at 298 K. The molarity of the solution is M. The osmotic pressure of the solution is atmospheres.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:30
What is the correct term for living the most sustainable life you can within your current circumstances?
Answers: 1

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 22.06.2019 14:50
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2
You know the right answer?
The nonvolatile, nonelectrolyte cholesterol, C27H46O (386.60 g/mol), is soluble in benzene C6H6. Cal...
Questions


Physics, 17.12.2020 18:20



Mathematics, 17.12.2020 18:20




Mathematics, 17.12.2020 18:20

Mathematics, 17.12.2020 18:20

Mathematics, 17.12.2020 18:20

Social Studies, 17.12.2020 18:20

Mathematics, 17.12.2020 18:20

Computers and Technology, 17.12.2020 18:20

Mathematics, 17.12.2020 18:20


Chemistry, 17.12.2020 18:20

Chemistry, 17.12.2020 18:20

Mathematics, 17.12.2020 18:20