Chemistry, 17.09.2019 06:00 markitakimbrough69
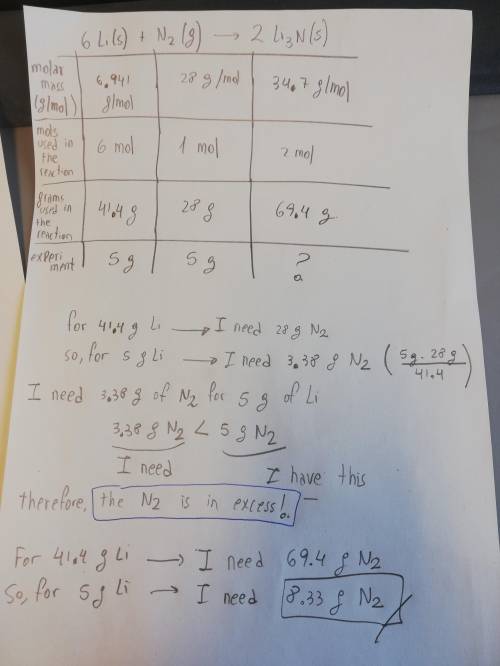
Lithium and nitrogen react in a combination reaction to produce lithium nitride: . 6li(s)+n2(g)→2li3n(s). in a particular experiment, 5.00-g samples of each reagent are reacted. the theoretical yield of lithium nitride is g.

Answers: 1
Another question on Chemistry

Chemistry, 23.06.2019 00:50
What is the enthalpy of combustion (per mole) of c4h10 (g)? –2,657.5 kj/mol –5315.0 kj/mol –509.7 kj/mol –254.8 kj/mol
Answers: 1

Chemistry, 23.06.2019 09:10
In a 28 g serving of cheese curls there are 247mg of sodium. how much sodium is in a 12.5 ounce bag
Answers: 1

Chemistry, 23.06.2019 14:30
William has eight more nickels than dimes in his pocket for a total of $2.50. which equation could be used to determine the number of x dimes in his pocket?
Answers: 1

Chemistry, 23.06.2019 15:30
The gas in a sealed container has an absolute pressure of 9.25 atmospheres. if the air around the container is at standard pressure, what is the gauge pressure inside the container
Answers: 1
You know the right answer?
Lithium and nitrogen react in a combination reaction to produce lithium nitride: . 6li(s)+n2(g)→2li3...
Questions

Biology, 21.08.2019 08:50

Mathematics, 21.08.2019 08:50

Mathematics, 21.08.2019 08:50


Geography, 21.08.2019 08:50



History, 21.08.2019 08:50

Mathematics, 21.08.2019 08:50






Mathematics, 21.08.2019 08:50

History, 21.08.2019 08:50

Geography, 21.08.2019 08:50



Social Studies, 21.08.2019 08:50