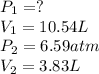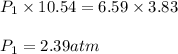Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1

Chemistry, 22.06.2019 10:00
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1
You know the right answer?
The volume of a sample of hydrogen gas was decreased from 10.54 L to 3.83 L at constant temperature....
Questions


Geography, 11.11.2020 22:50





Arts, 11.11.2020 22:50



Chemistry, 11.11.2020 22:50

Physics, 11.11.2020 22:50


Mathematics, 11.11.2020 22:50


Mathematics, 11.11.2020 22:50

Mathematics, 11.11.2020 22:50

Mathematics, 11.11.2020 22:50

English, 11.11.2020 22:50

Mathematics, 11.11.2020 22:50

Chemistry, 11.11.2020 22:50

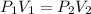
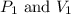 are initial pressure and volume.
are initial pressure and volume.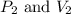 are final pressure and volume.
are final pressure and volume.