
Chemistry, 06.03.2020 23:26 lilquongohard
You carefully weigh out 16.00 g of CaCO3 powder and add it to 64.80 g of HCl solution. You notice bubbles as a reaction takes place. You then weigh the resulting solution and find that it has a mass of 74.24 g . The relevant equation is CaCO_3(s) + 2HCl(aq) rightarrow H_2O(l) + CO_2(g) + CaCl_2(aq) Assuming no other reactions take place, what mass of CO_2 was produced in this reaction?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Match the following items. 1. high-intensity bundle of energy being emitted from some decaying nuclei gamma ray 2. particle radiating from the nucleus of some atoms beta particle 3. negative particle identical to an electron but radiating from a decaying nucleus alpha particle
Answers: 1

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1


Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1
You know the right answer?
You carefully weigh out 16.00 g of CaCO3 powder and add it to 64.80 g of HCl solution. You notice bu...
Questions


Mathematics, 03.03.2021 02:00

Business, 03.03.2021 02:00

Mathematics, 03.03.2021 02:00

Social Studies, 03.03.2021 02:00


Chemistry, 03.03.2021 02:00

Mathematics, 03.03.2021 02:00

Mathematics, 03.03.2021 02:00

Mathematics, 03.03.2021 02:00

Mathematics, 03.03.2021 02:00

Arts, 03.03.2021 02:00


Mathematics, 03.03.2021 02:00

Social Studies, 03.03.2021 02:00

Advanced Placement (AP), 03.03.2021 02:00

Social Studies, 03.03.2021 02:00



Mathematics, 03.03.2021 02:00

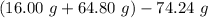
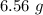
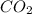 produced in this reaction was 6.56 grams
produced in this reaction was 6.56 grams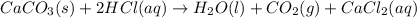
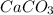 + mass of
+ mass of 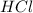 = 16.00 + 64.80 = 80.80 g
= 16.00 + 64.80 = 80.80 g

