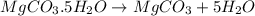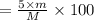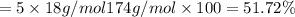You are in space and running out of water. You do have a great deal of magnesium carbonate pentahydrate. It is possible to extract the water from this. Determine the percent of water by mass in the hydrate magnesium carbonate pentahydrate (MgCO_3 middot 5H_2O). If your answer is 17%, enter 17, do not put in the percent sign. As an introduction to his class, Severus Snape teaches the first years at Hogwarts about hydrates. He begins with equations representing various dehydrations. Which of the following equations properly represents the dehydration of magnesium carbonate pentahydrate (MgCO_3 middot 5H_2O)?
MgCO_3 middot H_2O MgCO_3 + H_2O MgCO_3 middot 5H_2O
MgCO_3 + 5H_2O MgCO_3 middot 5H_2O MgCO_3 + H_2O
MgCO_3 middot 5H_2O MgCO_3 + 5H_2O

Answers: 3
Another question on Chemistry



Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 22:40
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1
You know the right answer?
You are in space and running out of water. You do have a great deal of magnesium carbonate pentahydr...
Questions

English, 18.03.2021 01:30

History, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30



Mathematics, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30

Health, 18.03.2021 01:30






Mathematics, 18.03.2021 01:30

History, 18.03.2021 01:30

Arts, 18.03.2021 01:30

History, 18.03.2021 01:30


Mathematics, 18.03.2021 01:30