

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2

Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1

Chemistry, 22.06.2019 15:30
Light waves can move through , but they travel fastest when they move through a(n) .
Answers: 1

Chemistry, 23.06.2019 06:00
Robert leaves a chocolate bar in his car while attending school all day. when he goes to his car in the afternoon, the bat has changed into gooey liquid. what happened to the chocolate bar
Answers: 1
You know the right answer?
The voltage generated by the zinc concentration cell described by the line notation Zn ( s ) ∣ ∣ Zn...
Questions

History, 08.02.2021 21:30

Computers and Technology, 08.02.2021 21:30


English, 08.02.2021 21:30

English, 08.02.2021 21:30

World Languages, 08.02.2021 21:30

Chemistry, 08.02.2021 21:30


History, 08.02.2021 21:30


Chemistry, 08.02.2021 21:30

English, 08.02.2021 21:30

History, 08.02.2021 21:30

Mathematics, 08.02.2021 21:30


Mathematics, 08.02.2021 21:30


Computers and Technology, 08.02.2021 21:30

Mathematics, 08.02.2021 21:30

Mathematics, 08.02.2021 21:30

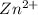 ion at cathode is 0.704 M
ion at cathode is 0.704 M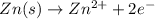
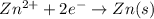
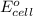 will be equal to zero.
will be equal to zero.![E_{cell}=E^o_{cell}-\frac{0.0592}{n}\log \frac{[Zn^{2+}_{anode}]}{[Zn^{2+}_{cathode}]}](/tpl/images/0536/7525/d92a3.png)
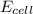 = 25.0 mV = 0.025 V (Conversion factor: 1 V = 1000 mV)
= 25.0 mV = 0.025 V (Conversion factor: 1 V = 1000 mV)![[Zn^{2+}_{cathode}]](/tpl/images/0536/7525/b27eb.png) = ? M
= ? M![[Zn^{2+}_{anode}]](/tpl/images/0536/7525/66f39.png) = 0.100 M
= 0.100 M![0.025=0-\frac{0.0592}{2}\log \frac{0.100}{[Zn^{2+}_{anode}]}](/tpl/images/0536/7525/4f785.png)
![[Zn^{2+}_{anode}]=0.704M](/tpl/images/0536/7525/377d9.png)


