
Chemistry, 07.03.2020 00:25 luisgonz5050
If 0.220 mol of solid CaCO3 and 670 mL of 0.433 M aqueous H2SO4 are reacted stoichiometrically according to the balanced equation. How many grams of solid CaSO4 are produced?
H2SO4(aq) + CaCO3(s) → CO2(g) + CaSO4(s) + H2O(l)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:00
What is driving behind plate tectonics (plate movment)? a) gravity only b) inertia c) convection and gravity d) the sun theres no option for science so i picked chemistry. plz
Answers: 2

Chemistry, 22.06.2019 12:00
Marcel just purchased 1.69 grams of iron fillings in order to make living putty for his 6 year old niece. how many moles of iron are made in his sample?
Answers: 1

Chemistry, 22.06.2019 17:50
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. start this question by first balance the chemical equation.1.) balance the equation: - alo3(s)+naoh(l)+hf(> na3alf6+h2o(g). 2.) if 17.5 kilograms of al2o3(s), 51.4 kilograms of naoh(l), and 51.4 kilograms of hf(g) react completely, how many kilograms of cryolite will be produced? 3.)which reactants will be in excess, (al2o3, naoh, or hf) 4.)what is the total mass of the excess reactants left over after the reaction is complete in kg?
Answers: 2

Chemistry, 22.06.2019 23:30
Rank the following four acids in order of increasing bronsted acidity : h2f+ , ch3oh, (ch3)2oh+ , ch3sh2+
Answers: 3
You know the right answer?
If 0.220 mol of solid CaCO3 and 670 mL of 0.433 M aqueous H2SO4 are reacted stoichiometrically accor...
Questions


History, 16.09.2021 20:00





World Languages, 16.09.2021 20:00

Mathematics, 16.09.2021 20:00




Computers and Technology, 16.09.2021 20:00



Mathematics, 16.09.2021 20:00



Mathematics, 16.09.2021 20:00


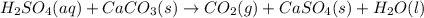
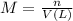
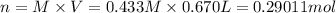
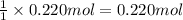 sulfuric acid.
sulfuric acid.

