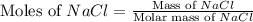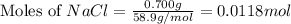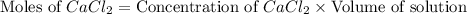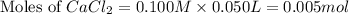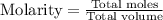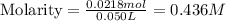Chemistry, 07.03.2020 00:21 dwilburn01
A solution is prepared by adding 0.700 g of solid NaClNaCl to 50.0 mL of 0.100 M CaCl2CaCl2. What is the molarity of chloride ion in the final solution? Assume that the volume of the final solution is 50.0 mL.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 04:30
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3

Chemistry, 22.06.2019 07:40
The formation of a solid, also known as a is an indication of a chemical change. precipitate particulate particle powder
Answers: 3

Chemistry, 22.06.2019 16:00
How do dying stars contribute to the formation of planets
Answers: 1
You know the right answer?
A solution is prepared by adding 0.700 g of solid NaClNaCl to 50.0 mL of 0.100 M CaCl2CaCl2. What is...
Questions

Chemistry, 24.08.2019 03:50


Chemistry, 24.08.2019 03:50

Mathematics, 24.08.2019 03:50


Geography, 24.08.2019 03:50

Mathematics, 24.08.2019 03:50


Physics, 24.08.2019 03:50

Mathematics, 24.08.2019 03:50


Mathematics, 24.08.2019 03:50

History, 24.08.2019 03:50



Mathematics, 24.08.2019 03:50

Mathematics, 24.08.2019 03:50


Mathematics, 24.08.2019 03:50

Social Studies, 24.08.2019 03:50

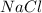 and
and 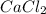 .
.