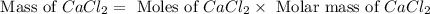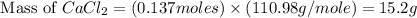Chemistry, 07.03.2020 00:15 IBillRandomz2958
When calcium carbonate is added to hydrochloric acid, calcium chloride, carbon dioxide, and water are produced. CaCO3 ( s ) + 2 HCl ( aq ) ⟶ CaCl2 ( aq ) + H 2 O ( l ) + CO 2 ( g ) How many grams of calcium chloride will be produced when 30.0 g of calcium carbonate is combined with 10.0 g of hydrochloric acid

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Write the balanced equation for a reaction between aqueous nitric acid (hno3) and solid lithium metal (this is a single replacement reaction)
Answers: 1

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

You know the right answer?
When calcium carbonate is added to hydrochloric acid, calcium chloride, carbon dioxide, and water ar...
Questions




















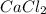 produced is, 15.2 grams.
produced is, 15.2 grams. = 30.0 g
= 30.0 g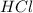 = 10.0 g
= 10.0 g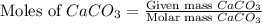
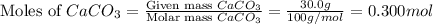
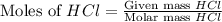
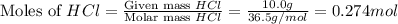
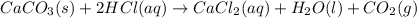
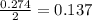 moles of
moles of