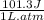Chemistry, 07.03.2020 00:09 makennskyee7114
Consider an ideal gas enclosed in a 1.00 L container at an internal pressure of 10.0 atm. Calculate the work, w , if the gas expands against a constant external pressure of 1.00 atm to a final volume of 10.0 L.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3


Chemistry, 22.06.2019 19:10
Which statement correctly describes the phosphate ion, ? it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge on the phosphorus atom. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge on the phosphorus atom.
Answers: 3

Chemistry, 22.06.2019 23:10
Amines are good nucleophiles, even though they are neutral molecules. how would the rate of an sn2 reaction between an amine and an alkyl halide be affected if the polarity of the solvent is increased? amines are good nucleophiles, even though they are neutral molecules. how would the rate of an reaction between an amine and an alkyl halide be affected if the polarity of the solvent is increased? because both reactants in the rate-limiting step are neutral, the reaction will be faster if the polarity of the solvent is increased. because both reactants in the rate-limiting step are neutral, the reaction will be slower if the polarity of the solvent is increased. because both reactants in the rate-limiting step are neutral, the reaction will occur at the same rate if the polarity of the solvent is increased. request answer
Answers: 3
You know the right answer?
Consider an ideal gas enclosed in a 1.00 L container at an internal pressure of 10.0 atm. Calculate...
Questions


Chemistry, 25.02.2021 17:40

Mathematics, 25.02.2021 17:40


Mathematics, 25.02.2021 17:40

Mathematics, 25.02.2021 17:40

Mathematics, 25.02.2021 17:40

Health, 25.02.2021 17:40

Mathematics, 25.02.2021 17:40



Mathematics, 25.02.2021 17:40




Mathematics, 25.02.2021 17:40




Physics, 25.02.2021 17:40