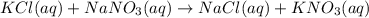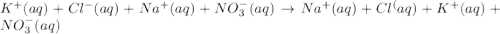Chemistry, 07.03.2020 00:02 raidattarab
An aqueous solution of potassium chloride is mixed with an aqueous solution of sodium nitrate. The complete ionic equation contains which of the following species (when balanced in standard form)?
a. Cl-(aq)
b. KNO3(aq)
c.3NO-3(aq)
d.2Na+(aq)
e.2K+(aq)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:30
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1

Chemistry, 22.06.2019 18:30
Read the claim. breakfast is an important meal. it jump starts the body’s process of using calories to break down food. appetite can decrease with age, but going too long without eating causes metabolism to slow down. current research shows that incorporating legumes such as lentils and chickpeas into meals boosts metabolism for twenty-four hours. who might benefit from this claim? people who have a fast metabolism stores that sell exercise equipment people who take vitamin supplements grocery stores that sell legumes
Answers: 1

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2

Chemistry, 23.06.2019 00:30
Fred is studying a substance that is made out of only one element. this means that
Answers: 1
You know the right answer?
An aqueous solution of potassium chloride is mixed with an aqueous solution of sodium nitrate. The c...
Questions


Mathematics, 22.04.2020 21:25

Physics, 22.04.2020 21:25





History, 22.04.2020 21:25

Mathematics, 22.04.2020 21:25

History, 22.04.2020 21:25


English, 22.04.2020 21:25

Physics, 22.04.2020 21:25

Mathematics, 22.04.2020 21:25

Health, 22.04.2020 21:25

Social Studies, 22.04.2020 21:25

Mathematics, 22.04.2020 21:25


Mathematics, 22.04.2020 21:25

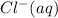 ions are present in the complete ionic equation
ions are present in the complete ionic equation