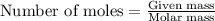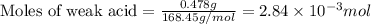Chemistry, 07.03.2020 00:03 smuindi3293
A student titrated 0.478 g of a weak acid with 0.217 M NaOH solution. How many moles of weak acid were in solution, if its molar mass was 168.45 g/mol

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3

Chemistry, 23.06.2019 04:10
Two solids are mixed in a flask and stirred. after a few minutes, the flask becomes cold. which of the following best describes this reaction? a. an exothermic reaction b. a combustion reaction c. an endothermic reaction d. a decomposition reaction
Answers: 1

Chemistry, 23.06.2019 05:50
What is the molecular formula of ferrous nitrate and ferric nitrate
Answers: 2
You know the right answer?
A student titrated 0.478 g of a weak acid with 0.217 M NaOH solution. How many moles of weak acid we...
Questions



Physics, 11.10.2020 23:01

Mathematics, 11.10.2020 23:01



Mathematics, 11.10.2020 23:01

History, 11.10.2020 23:01


Social Studies, 11.10.2020 23:01


Biology, 11.10.2020 23:01

Chemistry, 11.10.2020 23:01


Health, 11.10.2020 23:01


Social Studies, 11.10.2020 23:01

Mathematics, 11.10.2020 23:01

Physics, 11.10.2020 23:01

Arts, 11.10.2020 23:01

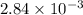 moles
moles