
Chemistry, 07.03.2020 00:34 Candieboo4006
Ammonium hydrogen sulfide decomposes according to the following reaction, for which Kp = 0.11 at 250ºC: NH4HS(s) H2S(g) + NH3(g) If 55.0 g of NH4HS(s) is placed in a sealed 5.0-L container, what is the partial pressure of NH3(g) at equilibrium

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 19:20
What is the strongest intermolecular force between an nacl unit and an h2o molecule together in a solution? covalent bonding dipole-dipole force hydrogen bonding ion-dipole force
Answers: 1

Chemistry, 22.06.2019 07:20
Which of these conditions most likely produces an unstable isotope?
Answers: 1

Chemistry, 22.06.2019 17:30
What will most likely happen in the absence of a cell membrane? a) photosynthesis will not take place. b) the cell will not store food, water, nutrients, and waste. c) energy will not be released during cellular respiration. d) substances will pass in and out of the cell in an uncontrolled manner.
Answers: 1

Chemistry, 22.06.2019 21:30
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3
You know the right answer?
Ammonium hydrogen sulfide decomposes according to the following reaction, for which Kp = 0.11 at 250...
Questions

Mathematics, 12.12.2020 21:00

Chemistry, 12.12.2020 21:00

SAT, 12.12.2020 21:00

History, 12.12.2020 21:00



Mathematics, 12.12.2020 21:00





Mathematics, 12.12.2020 21:00

Mathematics, 12.12.2020 21:00




Mathematics, 12.12.2020 21:00

Advanced Placement (AP), 12.12.2020 21:10


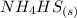 ⇄
⇄ 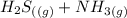
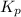 = 0.11
= 0.11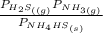
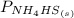 = 1 since it is solid and solid and it is known that any solid has uniformity at equilibrium.
= 1 since it is solid and solid and it is known that any solid has uniformity at equilibrium.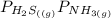
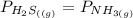
![[x][x]](/tpl/images/0536/8008/ce5d5.png)
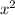
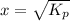
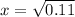
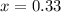 atm
atm

