
Chemistry, 07.03.2020 00:59 ElegantEmerald
An aqueous solution of sodium sulfide is allowed to react with an aqueous solution of magnesium chloride.
The complete ionic equation contains which of the following species (when balanced in standard form)?
a.
Cl-(aq)
b.
2Na+(aq)
c.
2S2-(aq)
d.
Na+(aq)
e.
3Mg2+(aq)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 22.06.2019 17:50
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3

Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3
You know the right answer?
An aqueous solution of sodium sulfide is allowed to react with an aqueous solution of magnesium chlo...
Questions





Mathematics, 20.07.2019 16:30



History, 20.07.2019 16:30

Mathematics, 20.07.2019 16:30


Mathematics, 20.07.2019 16:30


Mathematics, 20.07.2019 16:30


Mathematics, 20.07.2019 16:30


Biology, 20.07.2019 16:30

Social Studies, 20.07.2019 16:30

English, 20.07.2019 16:30

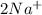
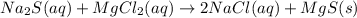
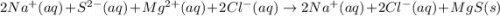
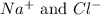 are the spectator ions.
are the spectator ions.

