

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:30
In an energy pyramid, which level has the most available energy?
Answers: 1

Chemistry, 22.06.2019 05:30
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3

Chemistry, 22.06.2019 13:30
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1

You know the right answer?
The rate constant of a certain reaction is known to obey the Arrhenius equation, and to have an acti...
Questions

History, 29.08.2019 16:30



Mathematics, 29.08.2019 16:30

English, 29.08.2019 16:30

Mathematics, 29.08.2019 16:30


Social Studies, 29.08.2019 16:30


Biology, 29.08.2019 16:30

History, 29.08.2019 16:30

Chemistry, 29.08.2019 16:30

Health, 29.08.2019 16:30



Computers and Technology, 29.08.2019 16:30


Geography, 29.08.2019 16:30


History, 29.08.2019 16:30

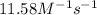
![\ln(\frac{K_{56.0^oC}}{K_{-8.0^oC}})=\frac{E_a}{R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0536/9043/5e447.png)
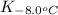 = equilibrium constant at -8.0°C =
= equilibrium constant at -8.0°C = 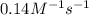
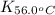 = equilibrium constant at 56°C = ?
= equilibrium constant at 56°C = ?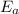 = Activation energy = 50.0 kJ/mol = 50000 J/mol (Conversion factor: 1 kJ = 1000 J)
= Activation energy = 50.0 kJ/mol = 50000 J/mol (Conversion factor: 1 kJ = 1000 J)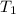 = initial temperature =
= initial temperature = ![-8.0^oC=[273-8]K=265K](/tpl/images/0536/9043/f4197.png)
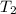 = final temperature =
= final temperature = ![56.0^oC=[273+56]K=329K](/tpl/images/0536/9043/23b22.png)
![\ln(\frac{K_{56.0^oC}}{0.14})=\frac{50000J}{8.314J/mol.K}[\frac{1}{265}-\frac{1}{329}]\\\\K_{56.0^oC}=11.58M^{-1}s^{-1}](/tpl/images/0536/9043/35497.png)


