Chemistry, 07.03.2020 01:27 Nevaeh3700
Consider the Fischer ester synthesis of methyl benzoate from benzoic acid and methanol in the presence of sulfuric acid as a catalyst. A reaction was performed in which 3.8 g of benzoic acid was reacted with excess methanol to make 1.8 g of methyl benzoate. Calculate the theoretical yield and percent yield for this reaction.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:00
When a comet collides with earth, it adds material to our planet and causes great damage. therefore, a collision like this is a a. destructive force b. constructive force c. geologic process and event d. constructive and destructive force
Answers: 1

Chemistry, 21.06.2019 22:30
Using the periodic table, complete the table to describe each atom. type in your answers.a ? b? c? d? e? f?
Answers: 1


Chemistry, 22.06.2019 04:30
In which phase(s) do the molecules take the shape of the container?
Answers: 1
You know the right answer?
Consider the Fischer ester synthesis of methyl benzoate from benzoic acid and methanol in the presen...
Questions

English, 16.06.2021 16:40

Chemistry, 16.06.2021 16:40

Mathematics, 16.06.2021 16:40

Mathematics, 16.06.2021 16:40


History, 16.06.2021 16:40


English, 16.06.2021 16:40

English, 16.06.2021 16:40




Chemistry, 16.06.2021 16:40







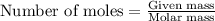 .....(1)
.....(1)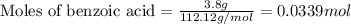
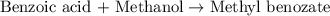
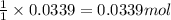 of methyl benzoate
of methyl benzoate