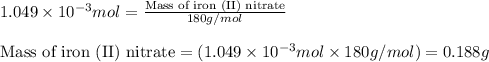Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
200. ml of 3.00 m nacl solution is diluted to a final volume of 500. ml. what is the molarity of the final solution?
Answers: 2

Chemistry, 22.06.2019 04:00
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2

Chemistry, 22.06.2019 07:00
The organism shown is a free-living one that is anchored to the bottom of ponds and streams during one stage of its life cycle what is the common name for the group to which this organism belong
Answers: 3

Chemistry, 22.06.2019 22:30
What methods could you use to solubilize calcium carbonate
Answers: 1
You know the right answer?
What minimum mass of iron (II) nitrate must be added to 10.0 of a 0.0699 M phosphate solution in ord...
Questions

Health, 05.10.2020 16:01

Geography, 05.10.2020 16:01


Mathematics, 05.10.2020 16:01


Mathematics, 05.10.2020 16:01

History, 05.10.2020 16:01


Physics, 05.10.2020 16:01

Mathematics, 05.10.2020 16:01

Mathematics, 05.10.2020 16:01


Mathematics, 05.10.2020 16:01


Mathematics, 05.10.2020 16:01




History, 05.10.2020 16:01

Mathematics, 05.10.2020 16:01

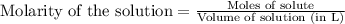

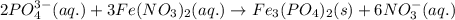
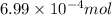 of phosphate solution will react with =
of phosphate solution will react with = 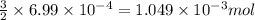 of iron (II) nitrate
of iron (II) nitrate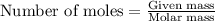
 moles
moles