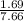Chemistry, 07.03.2020 02:47 garretthyatt123
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. An industrial chemist studying this reaction fills a 200. mL flask with 2.6 atm of sulfur dioxide gas and 0.90 atm of oxygen gas at 35.°C. She then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of sulfur trioxide gas to be 1.3 atm. Calculate the pressure equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. Round your answer to 2 significant digits.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 16:00
Based on the law of conservation of energy, which statement is false? answer- energy is lost when machines dont work right
Answers: 1

Chemistry, 22.06.2019 11:40
Effect of rotenone and antimycin a on electron transfer rotenone, a toxic natural product from plants, strongly inhibits nadh dehydrogenase of insect and fish mitochondria. antimycin a, a toxic antibiotic, strongly inhibits the oxidation of ubiquinol. (a) explain why rotenone ingestion is lethal to some insect and fish species. (b) explain why antimycin a is a poison. (c) given that rotenone and antimycin a are equally effective in blocking their respective sites in the electron-transfer chain, which would be a more potent poison? explain.
Answers: 3

Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 7.3 × 106 m/s. show your work. note: h = plank's constant (6.62607 x 10-34 j s)
Answers: 1

Chemistry, 23.06.2019 12:10
Which structure is a valid representation of a hydrocarbon molecule?
Answers: 2
You know the right answer?
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid...
Questions

Mathematics, 31.07.2019 21:30

Social Studies, 31.07.2019 21:30

History, 31.07.2019 21:30

English, 31.07.2019 21:30


Social Studies, 31.07.2019 21:30



Mathematics, 31.07.2019 21:30

Mathematics, 31.07.2019 21:30

Social Studies, 31.07.2019 21:30

History, 31.07.2019 21:30

Biology, 31.07.2019 21:30





Mathematics, 31.07.2019 21:30

History, 31.07.2019 21:30

Mathematics, 31.07.2019 21:30

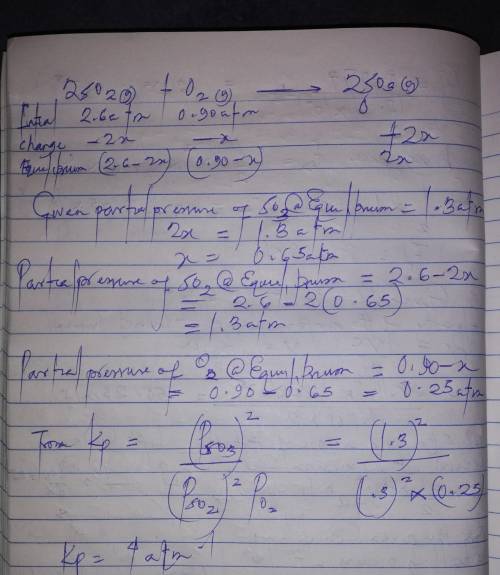
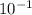 = 0.22 to 2 sig. fig.
= 0.22 to 2 sig. fig.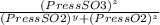
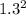 ÷ (
÷ (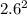 + 0.9)
+ 0.9)