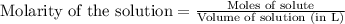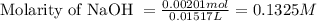Chemistry, 07.03.2020 02:37 loopysoop5035
A 0.411 g sample of potassium hydrogen phthalate, KHC8H4O4 (MM = 204.44 g/mol) is dissolved in 50 mL of deionized water in a 125-Erlenmeyer flask. The sample is titrated to the phenolphthalein endpoint with 15.17 mL of a sodium hydroxide solution. What is the molar concentration of the NaOH solution?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 19:30
Water molecules have a strong attraction to each other because of hydrogen bonding, allowing water to move against gravity up a plant's stem through capillary action. true false
Answers: 2

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 15:30
Why does earth rotate? because earth is formed from cold gases collapsing due to gravity because the matter in the nebula that formed earth was spinning because earth forms more than 99% of the mass of the solar system because the hydrogen atoms inside the nebula fused to form helium
Answers: 1
You know the right answer?
A 0.411 g sample of potassium hydrogen phthalate, KHC8H4O4 (MM = 204.44 g/mol) is dissolved in 50 mL...
Questions

Business, 03.08.2019 21:30

Health, 03.08.2019 21:30



Mathematics, 03.08.2019 21:30


Mathematics, 03.08.2019 21:30



Chemistry, 03.08.2019 21:30

Mathematics, 03.08.2019 21:30

English, 03.08.2019 21:30



Mathematics, 03.08.2019 21:30




History, 03.08.2019 21:30

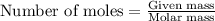
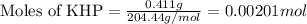
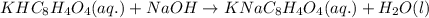
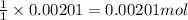 of NaOH.
of NaOH.