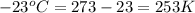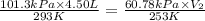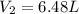Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 3

You know the right answer?
A helium balloon with an internal pressure of 1.0 atm and a volume of 4.50 L at 20.0⁰C is released....
Questions






Mathematics, 12.04.2021 08:00

Chemistry, 12.04.2021 08:00




English, 12.04.2021 08:00

History, 12.04.2021 08:00

Spanish, 12.04.2021 08:00


Mathematics, 12.04.2021 08:00


Mathematics, 12.04.2021 08:00


English, 12.04.2021 08:00

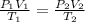
 = initial pressure of gas = 1 atm = 101.3 kPa
= initial pressure of gas = 1 atm = 101.3 kPa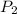 = final pressure of gas = 60.78 kPa
= final pressure of gas = 60.78 kPa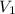 = initial volume of gas = 4.50 L
= initial volume of gas = 4.50 L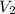 = final volume of gas = ?
= final volume of gas = ?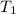 = initial temperature of gas =
= initial temperature of gas = 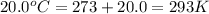
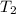 = final temperature of gas =
= final temperature of gas =