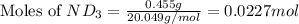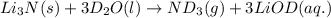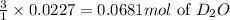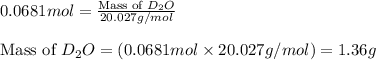Lithium nitride reacts with water to produce ammonia and lithium hydroxide according to the equation Li 3 N ( s ) + 3 H 2 O ( l ) ⟶ NH 3 ( g ) + 3 LiOH ( aq ) Heavy water is water with the isotope deuterium in place of ordinary hydrogen, and its formula is D 2 O . The same reaction can be used to produce heavy ammonia, ND 3 ( g ) , according to the equation Li 3 N ( s ) + 3 D 2 O ( l ) ⟶ ND 3 ( g ) + 3 LiOD ( aq ) Calculate how many grams of heavy water are required to produce 455.0 mg ND 3 ( g ) . The mass of deuterium, D , is 2.014 g/mol.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 1

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 09:00
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3

Chemistry, 22.06.2019 12:00
What is a possible quantum number set for an electron in the 3s orbital of a magnesium atom
Answers: 1
You know the right answer?
Lithium nitride reacts with water to produce ammonia and lithium hydroxide according to the equation...
Questions

Mathematics, 04.06.2020 14:05

History, 04.06.2020 14:05



Mathematics, 04.06.2020 14:05

History, 04.06.2020 14:05

Mathematics, 04.06.2020 14:05

Physics, 04.06.2020 14:05

Mathematics, 04.06.2020 14:05


Business, 04.06.2020 14:05

Mathematics, 04.06.2020 14:05

Mathematics, 04.06.2020 14:05

History, 04.06.2020 14:05


Mathematics, 04.06.2020 14:05

Mathematics, 04.06.2020 14:05



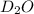 needed is 1.36 grams
needed is 1.36 grams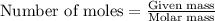 .....(1)
.....(1)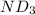 :
: