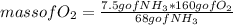Using the following reaction (depicted using molecular models), large quantities of ammonia are burned in the presence of a platinum catalyst to give nitric oxide as the first step in the preparation of nitric acid. Suppose a vessel contains 7.50 g of NH3, how many grams of O2 are needed for a complete reaction?4 NH3 + 5 O2 > 4 NO + 6 H2O

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:40
Does the energy in a solid increase or decrease when changing to a liquid?
Answers: 1

Chemistry, 21.06.2019 18:30
150.0 grams of asf3 were reacted with 180.0 g of ccl4 to produce ascl3 and ccl2f2. if the actual yield of ccl2f2 was 127 g, what is the percent yield?
Answers: 2

Chemistry, 22.06.2019 04:50
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

Chemistry, 22.06.2019 22:30
What must be in balance for temperatures to remain constant?
Answers: 1
You know the right answer?
Using the following reaction (depicted using molecular models), large quantities of ammonia are burn...
Questions

Mathematics, 14.07.2020 01:01



Chemistry, 14.07.2020 01:01

English, 14.07.2020 01:01

Computers and Technology, 14.07.2020 01:01

Mathematics, 14.07.2020 01:01

Mathematics, 14.07.2020 01:01

Mathematics, 14.07.2020 01:01



Mathematics, 14.07.2020 01:01





Mathematics, 14.07.2020 01:01


Mathematics, 14.07.2020 01:01

Mathematics, 14.07.2020 01:01