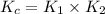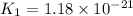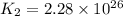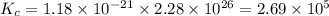Consider the following reactions and their equilibrium constants at 303 K. PCl5(g) equilibrium reaction arrow 1/4 P4(g) + 5/2 Cl2(g); Kc = 1.18 ✕ 10−21 1/4 P4(g) + 3/2 Cl2(g) equilibrium reaction arrow PCl3(g); Kc = 2.28 ✕ 1026 Calculate Kc for PCl5(g) equilibrium reaction arrow PCl3(g) + Cl2(g) at the same temperature.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Joseph has hypothesized that sound travels in waves. if he were following the scientific method, what should he do next? a. ask a question. b. test the hypothesis. c. study the results. d. tell other scientists about his hypothesis.
Answers: 1

Chemistry, 22.06.2019 03:30
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3

Chemistry, 22.06.2019 13:50
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3

Chemistry, 22.06.2019 15:00
Answer explain why it is not possible to deduce a complete order of reactivity.
Answers: 3
You know the right answer?
Consider the following reactions and their equilibrium constants at 303 K. PCl5(g) equilibrium react...
Questions

Mathematics, 04.06.2020 13:22





Mathematics, 04.06.2020 13:22



Mathematics, 04.06.2020 13:22

Mathematics, 04.06.2020 13:22





Mathematics, 04.06.2020 13:22


Chemistry, 04.06.2020 13:22


Mathematics, 04.06.2020 13:22

Mathematics, 04.06.2020 13:22

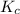 for the net reaction is
for the net reaction is 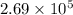
![PCl_5(g)\xrightarrow[]{K_1} \frac{1}{4}P_4(g)+\frac{5}{2}Cl_2(g)](/tpl/images/0537/3407/f38c5.png)
![\frac{1}{4}P_4(g)+\frac{3}{2}Cl_2(g)\xrightarrow[]{K_2} PCl_5(g)](/tpl/images/0537/3407/948a1.png)
![PCl_5(g)\xrightarrow[]{K_c} PCl_3(g)+Cl_2(g)](/tpl/images/0537/3407/3c32f.png)